|
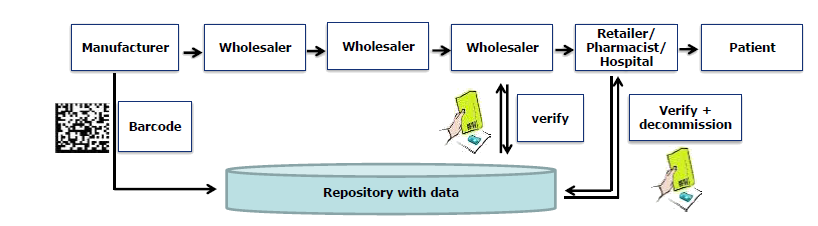
In order to prevent falsification and counterfeiting of medicines, worldwide regulations are created to provide packages of medicines with a unique number that is registered centrally. In the meantime, European regulations are in force and therefore additional protection on packaging of medicines with a 2D Data Matrix code is mandatory. Track & Trace legislation is also active in other countries, such as the USA and Brazil. In addition, legislation is being considered to enable the packaging to be traced in addition to this unique number. This is done by a Track and Trace (serialization and aggregation) technique. All this has a major impact on all companies dealing with medicines.
The following subjects will at least be covered:
Who should attend this training This training is specially designed for Qualification- Validation staff, Quality Assurance, Engineers, ICT employees, Project Managers, Process Packaging Engineers and others who are interested in Serialisation and Aggregation technics and methods. If non-Dutch students participate, the training will be in English language. General information
Trainers Price
Registration You can register for this training using the tab 'Training schedule'. For information please contact Marcel Schakel, phone +31 (0) 6 49022609 or email info@PQTInternational.com |